Publications
- Home
- Publications
- Oligonucleotide Purification by Ion Exchange Chromatography : Step-by-Step Guide to Process Understanding, Modeling, and Simulation
Oligonucleotide Purification by Ion Exchange Chromatography : Step-by-Step Guide to Process Understanding, Modeling, and Simulation
Published in OPRD in collaboration with GSK, AstraZeneca and Johnson & Johnson
- by Ypso-Facto
- April 12, 2024
Check out Ypso-Facto's new publication on oligonuclotide purification by Ion Exchange Chromatography in collaboration with GSK, Astrazeneca and Johnson & Johnson.
ABSTRACT
Oligonucleotides have emerged as a promising class of pharmaceuticals, leading to significantly increased demand.
Oligonucleotides are typically produced by solid-phase synthesis and then purified by ion exchange or reverse-phase chromatography. Predictive simulation is a valuable tool to help reduce process development times, secure scale-up, and decrease waste generation.
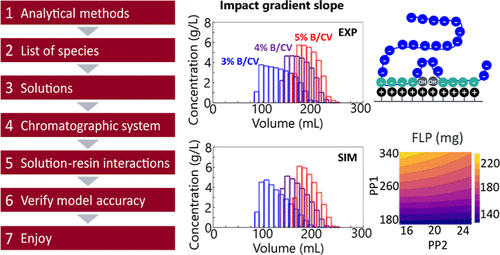
In this paper, we disclose for the first time a cutting-edge mechanistic model describing oligonucleotide purification by ion exchange chromatography. The novel aspect of the model and focus of this paper is the thermodynamic description of large, highly charged molecules, which includes both solution chemistry and the ion exchange mechanism with the chromatographic medium. The different retention of such molecules depending on their sequence length, charge state, and interaction strength with the resin is accurately predicted.
Thanks to a meaningful description of the underlying physical and chemical phenomena, the model also has highly predictive capabilities outside of the experimentally studied parameter ranges. It can be used to predict the outcome of changes to the operating conditions and experimental protocol, like the pH or ionic strength of buffer solutions, the number of washing steps, the loaded sample quantity, and more.
The model can also account for a change of configuration from a single column to a multicolumn system. The step-by-step methodology to implement this model is presented and illustrated with examples from three leading pharmaceutical companies in the field.
This methodology has been shown to lead to a significant process understanding with minimal experimental effort.
Click here to read the full article in OPRD
AUTHORS INFORMATION
- Kilian Kobl, Lucrèce Nicoud, Edouard Nicoud − Ypso-Facto, 54000 Nancy, France;
- Anna Watson, John Andrews, Edward A. Wilkinson, Muhid Shahid − Chemical Development, Pharmaceutical Technology & Development, Operations, AstraZeneca, Macclesfield SK10 2NA, U.K.;
- Christopher McKay, Benjamin I. Andrews, Batool Ahmed Omer, Olga Narducci, Edward Masson, Suzanne H. Davies − Chemical Development, GSK, Stevenage SG1 2NY, U.K. ;
- Tobias Vandermeersch − Johnson and Johnson, 2340 Beerse, Belgium.
Recent publications
- April 12, 2024
Oligonucleotide Purification by Ion Exchange Chromatography : Step-by-Step Guide to Process Understanding, Modeling, and Simulation - March 07, 2024
Bioprocess Simulation: Why the Industry Needs To Embrace Engineering Rather Than Empirical Approaches? - April 20, 2023
Purification of Protein by HIC

Ypso-Facto is a service company helping industrial firms to develop, optimize and secure their chemical processes and bioprocesses.
Download
Contact Us
-
Address: 19 avenue Foch
54000 NANCY - FRANCE - Phone: +33(0) 355 961 650
- Email: contact@ypso-facto.com